TABLE OF CONTENTS
Numerous individuals have joined the trend of using CBD oil for its theraputic benefits. When taking CBD it is important to know how much to take.
In this article, we take a look at how to calculate your dose, as well as the answers to some frequently asked questions. Always consult with your doctor.
What is CBD Oil?
CBD, short for cannabidiol, is a natural compound found in the cannabis plant. Unlike THC, another compound found in cannabis, CBD does not produce a psychoactive effect or a “high.”
It interacts with the body's endocannabinoid system, which is responsible for regulating various functions such as sleep, mood, appetite, and pain.
CBD is commonly used for its potential therapeutic benefits, such as reducing anxiety and inflammation, improving sleep quality, and easing chronic pain.
It can be extracted from hemp or marijuana plants and can be consumed in various forms, including oil tinctures, capsules, gummies, and topicals. Despite its growing popularity, CBD is not yet regulated by the FDA, so it's important to do your research and buy from reputable sources.
CBD Dosage Chart General Guidelines
When it comes to determining the correct dosage of CBD oil, it's important to consider a variety of factors. There is no one-size-fits-all answer. These factors may include your body weight, the severity of your symptoms, and the specific product you're using.
Generally, it's recommended that beginners start with a low dose (around 5-10mg) and gradually increase as needed until desired results are achieved.
Taking 20mg of CBD oil a day is a good start. If you don’t feel any effects, you can up the amount. Most CBD oils come in a dropper bottle. Each drop usually contains 5mg of CBD oil but that depends on the type and brand.
How Much CBD Should I Take The First Time?
Start with as little as you can. If you have CBD oil in a dropper bottle, then start with one drop. If you have CBD capsules, you can start with one capsule. Record the amount of CBD you took, what time you took it, and when you started feeling the effects.
This can help you study the effects of it on your body. Keeping track of these statistics can help you determine if you need to increase your dose.
What Can CBD Oil Be Mixed With?
CBD oil can be incorporated into various foods and drinks to mask its smell and taste. You can add CBD oil to your morning coffee, tea, smoothies, or take it directly under your tongue. CBD oil also comes in capsules and gummies, which makes it easier to eat.
When Should I Take CBD oil?
If you want to use CBD oil to deal with pain and anxiety during the day, you will benefit from having CBD oil with your morning coffee. Or, you can take CBD oil at night for a calm and mellow night's sleep.
Easy CBD Dosage Calculator
How can you calculate the dose of CBD that you need to take? This section will take you through the steps you need to take to find the most accurate dose explicitly tailored for you.
For this, you will need to know your weight and the strength of CBD oil that you want to take for your individual needs.
CBD Oil Strength Dosing
Learning the strength and potency of CBD oil can be difficult when you are just starting, but you should keep in mind that there is no such thing as overdosing on CBD.
CBD may lead to diarrhea or other gastrointestinal issues, but studies show that taking a lot of CBD won't lead to adverse effects, unlike other dietary supplements or medications. That said, here are the doses to consider when taking CBD oil:
- Low strength: 1mg of CBD per 10 pounds or 0.1mg
- Medium strength: 3mg of CBD per 10 pounds or 0.3mg
- High strength: 6mg of CBD per 10 pounds or 1.6mg
Calculate CBD Potency In CBD Oil
Many people are interested in determining the potency of CBD in their CBD oil. To calculate this, all you have to do is read the product details and get these two figures:
- Amount on CBD listed on the bottle in mg
- Bottle size in ml
To calculate CBD potency in CBD oil:
CBD in mg / bottle size in ml = CBD potency
CBD Oil Recommended Dosage
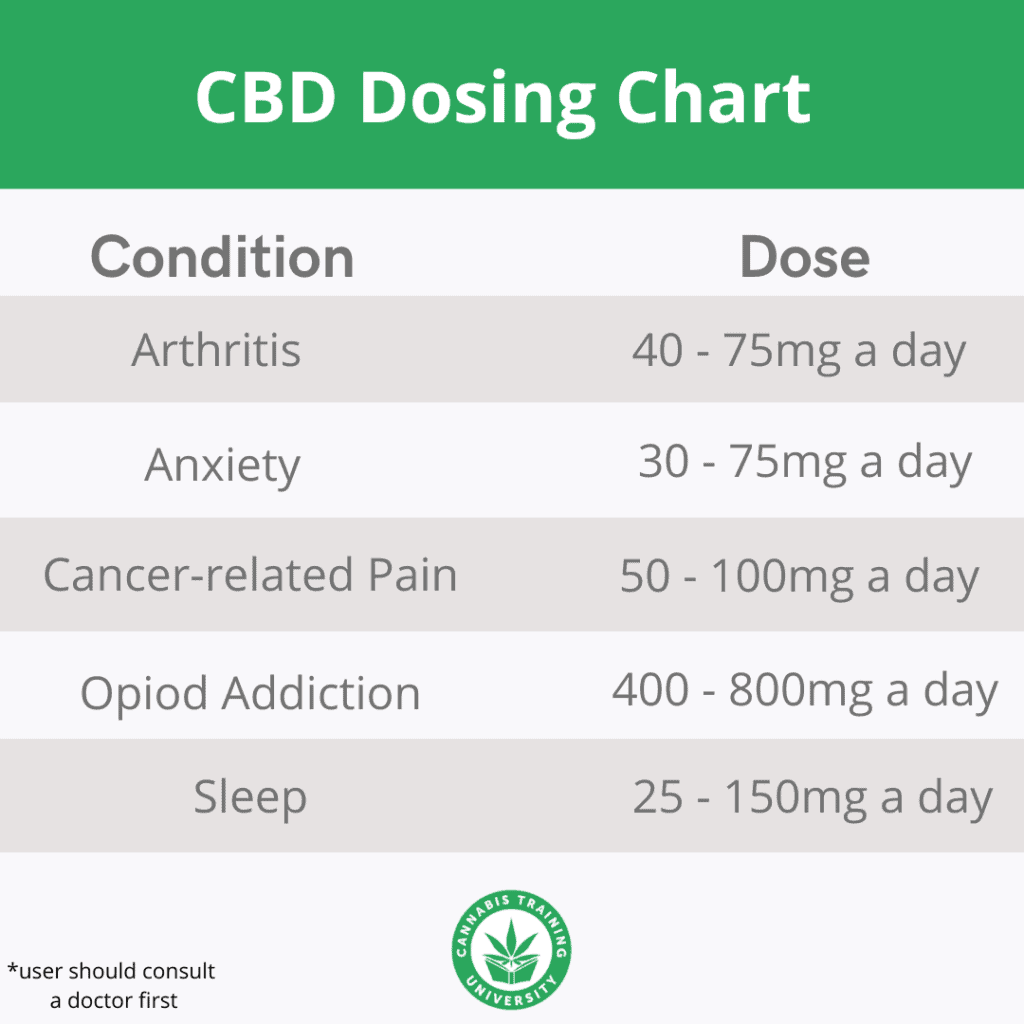
How much CBD can you take in one day? Before starting your CBD oil journey, you need to determine what condition you are targeting with your CBD use. CBD is excellent for anxiety and depression relief, along with improving sleep and reducing pain.
Other people take CBD oil for regular everyday use to boost their health. Let’s take a look at how much CBD to take for different conditions.
CBD Dosage For Everyday Use
CBD oil is a dietary supplement that people take to boost their general health, whether that is by providing calm or reducing anxiety. For such users, what is the correct dosage for everyday CBD?
Lower to moderate amounts of CBD is ideal for daily usage.
Some users report that they reap CBD oil benefits from as little as 1mg a day. For regular everyday use, it's a good idea to experiment to figure out what works best for you.
Start with 20mg of CBD oil, one CDB capsule, or one CBD gummy. If you don’t feel much of a difference, you can keep upping the dose until you feel a positive result.
CBD Dosage For Anxiety
Many people love to take CBD oil to combat anxiety. How much CBD for anxiety is ideal?
Moderate to high amounts of CBD is perfect for reducing anxiety and stress. This would amount to 25-75mg of CBD oil.
If you need more anxiety relief from CBD oil, you can consider upping the dose or the frequency with which you consume CBD. Note that high amounts of CBD oil from 1500-2000mg have been tested on humans with no adverse effects. Tailor your CBD dosage to your needs.
CBD Dosage For Depression
Research shows that CBD latches onto the brain’s serotonin receptors and influences the way they work. Low serotonin levels have been shown to lead to depression.
However, the causes of depression may vary from person to person.
It is for this reason that the CBD dosage for depression may take some experimentation. It’s advisable to start with smaller doses of CBD oil to help your body to adapt to it.
For depression, use 10mg of CBD oil and work up to 25mg or more.
CBD Dosage For Pain
When taking CBD oil for pain, you need to consider the level of pain you are experiencing. Mild low back pain and arthritis can be treated with a lower dose of 20 mg CBD oil.
However, many people seeking to use CBD oil usually experience severe pain, for example, arthritis, nerve pain, multiple sclerosis, and chronic pain. For such people, doses from 25mg of CBD oil and more will be helpful.
CBD oil may take a few minutes or hours to take effect and provide pain relief.
CBD Dosage For Sleep
Users report that CBD oil improves their quality of sleep. Research shows that people who took 25mg of CBD regularly had improved sleep after the first month.
Based on this research, it is safe to say that 25mg of CBD oil will improve sleep.
Taking a melatonin-infused CBD oil is also suitable for promoting relaxation.
Other CBD Products Dosage
CBD oil is not the only method of consuming cannabidiol. There are some other alternatives to CBD oil (which might taste bitter to some.) CBD gummies and edibles are a fun way to reap the benefits of CBD, like pain and anxiety relief. Here are the doses for other CBD products:
CBD Gummies Dosage
CBD gummies are easy to dose because they come in standard sizes. Most CBD gummies come in concentrations of 11 – 60mg of CBD.
Your dosage depends on the strength of effects that you need. For first time users, it’s better to start with the smaller dose. If you suffer from chronic pain, you might want to try 60mg CBD gummies.
CBD Vape Dosage
CBD vapers tend to micro-dose CBD oil throughout the day to reap the therapeutic effects. For first time users, try one puff and study its impact over the course of one hour. If you need to increase the CBD potency, you can increase the number of inhales you take.
CBD Balm Dosage
CBD balms come in different strengths and potency. Regardless, trying a patch test before committing to using any skincare products is recommended. If you need to increase the effects of a CBD balm, then you can increase the frequency with which you apply it.
CBD Oil For Pets
CBD oil is all the rage right now, even in the pet world. Proud pet parents want to give their furry babies the best supplements, and nowadays, CBD oil is that supplement.
Pets suffer from pain, arthritis, and anxiety too. For these moments, CBD oil might be the medicine that you reach for. Let's take a look at the CBD dosing for dogs and cats.
CBD Oil Dosage For Dogs
Your canine buddy might be getting older, and they're in pain due to arthritis. For this, using CBD oil is a great way to give them some pain relief. Note that an overdose of CBD in dogs won't lead to any adverse effects.
The most that will happen is a loose stool or stomach upset. Your dog may also feel tired after taking CBD, but that will naturally subside after a few doses.
How much CBD oil should I give my dog?
Base your dog’s CBD dosage on its weight. A good rule of thumb is to administer 1mg per 10lbs. If your dog is 25lbs heavy, then a dose of 2.5mg per day is just perfect.
CBD Oil Dosage For Cats
Cats can also benefit from CBD oil. Older cats on CBD oil can feel an increase in appetite and playfulness.
If your cat gets spooked by fireworks, CBD oil can calm them down so they can have a relaxing night during the loud booms. Make sure to select only full-spectrum hemp oil to give your cat that shot of CBD.
The normal CBD dosage for cats would be around 4mg of CBD once a day. Of course, not all cats are the same size and weight. For that reason, you can administer the dose based on their weight. For every 10lbs in weight, administer 1mg of CBD oil a day.
Dosing Marijuana
Here is an updated guide on marijuana dosing to help direct your responsible cannabis consumption.
Whether you take cannabis for the occasional high or you are on it for medical reasons, knowing the right amount of cannabis to take is key.
Cannabis dosing can be a tricky venture as there are quite a number of moving parts such as varying chemical and cannabinoid potency, even within the same strain.

How can you best navigate this unknown terrain?
Let’s get started!
What Factors Contribute To The “Cannabis High”?
The “Cannabis high” is influenced by three factors:
- Cannabis Dosage – Which is the amount of cannabinoid you have consumed
- Self – Physiological make-up of different users.
- State – What emotional and mental state they happen to be in at the time of use.
Since we cannot control the factors that are specific to an individual, we will deal with the one we can control. Dosage.
Because each method of consumption impacts the body differently, dosages are tailor made to suit each method.
How To Read Cannabis Packaging Correctly
If you are among the lucky who can purchase cannabis legally, you will find that the vape cartridges, edibles and pre-packaged flower come with a label. These labels can be quite confusing if you don’t know what you’re looking for.
While trying to understand the label, always pay close attention to the potency/dose information.
Products such as vape cartridges sometimes outline the total cannabinoids (such as THC) found in the cartridge. Edibles on the other hand could include the suggested dose alongside the total cannabinoids contained in the pack.
If you are purchasing bud, the labels usually indicate the total cannabinoids as a percentage contained in the bud. You can then calculate the dosage you need by dividing the total weight of the bud by the dosage you need.
You can use the formula below as follows:
mg of dried bud × % THC (as a decimal)
If for example you want to roll a joint that is 500mg (0.5g) of a strain with 14% THC, you’d calculate the amount of THC in the in joint this way:
500mg × 0.14 = 70mg THC
Having covered the basics, let’s get into the dosage of each cannabis form:
1. How To Dose Dry Marijuana Herb
- Typical dose: 25–0.5g of dried bud
Smoking or vaping cannabis brings on a pretty fast onset. This is considered one of its advantages. Depending on your tolerance and the potency of strain, you should feel the effects 5-15 minutes after smoking. This is because the inhaled smoke or vapor which contains the cannabinoids and terpenes finds its way almost directly into the bloodstream through the lungs.
On average, burning up 0.25 – 0.5g of dry herb should give you the desired effect. This is pre-calculated to contain ideal amounts of THC which usually average about 14%.
When venturing out, you can start with 0.25 g of dried bud and work your way up to 0.5 g. Also, starting at a smaller dose will ensure that the weed does not knock you out if you happen to be smoking a particularly potent strain.
2. How To Dose Marijuana Edibles
- Typical dose: 10mg of CBD or THC
As mentioned earlier, if you purchase your edibles from a legal cannabis store or dispensary it will likely have clear dosage instructions indicated on it.
If your label gives the cannabinoid content in percentage form, you could then use the formula below to determine how much cannabinoid it contains and therefore what quantity you need to dose yourself correctly.
The total weight of the edible (in mg) × % cannabinoids
While cooking with cannabis, you might find it a little harder to calculate the amount of cannabinoids. That being the case, you need to keep track of the cannabinoids right from the start in the process of making your butter. Before you commence, take note of the content of cannabinoids in your flower. That should be calculating the cannabinoid content in your butter.
Say you use 28 grams (which is 1 ounce) of flower that has 18% THC in the making of 250g of butter, the total cannabinoids in your butter.
“28,000mg of flower × 0.18 (18%) THC = 5040mg of THC in 250,000mg (250g) of butter (2% THC)”
Now, imagine that you have a brownie recipe that will require you to use 150g of butter. You will end up with a batch of brownies that has a total of 3g THC (150g butter X 0.02% THC). By dividing the total amount of the batch by the portion you actually consume in one sitting, you can arrive at the amount of THC you will have consumed.
Most edibles have a single dosage of 10mg of cannabinoid. You might find instances where the cannabinoid content could get as high as 100mg, though this is not meant to be consumed all at once. If you are making your own cannabis edibles at home it is easier to keep track of and dose.
Effects of Marijuana Edibles
Unlike smoking and vaping, edibles usually take longer to manifest. Because edibles have to go through the digestion process before they reach the bloodstream, you might even feel the effects after 2 hours.
As such, it is best to wait for this time to elapse before you add onto your initial dose. Both novices and seasoned users are advised to take it slow because the effects can hit hard very hard.
3. How To Dose CBD Oil Extracts
- Typical dose: 10mg of CBD
CBD oil extracts are usually used for medicinal purposes. A typical dose would usually contain 10mg of CBD taken orally. This however may vary according to individual requirements.
Determining the amount of CBD per can get confusing because manufacturers use mg on their labels sometimes while they use percentages in other instances.
You could determine the correct dosage per drop by dividing the total CBD in the bottle by the drops, or finding the total CBD in the bottle by the formula given above for calculating percentages.
That is:
“The total weight of the edible (in mg) × % cannabinoids”
Once you get the amount of cannabinoids in the bottle, you can calculate how many drops you need to take to meet your dosage requirements.
It is vital that you purchase CBD oil with clearly labelled content so as to be able to calculate your dosage correctly. Also, ensure that it is pure and not adulterated.
4. How To Dose Full-Extract Marijuana Oil
- Typical dose: Up to 1g, taken in bits
Rick Simpson is an example of full-extract cannabis oils. They are both concentrated and highly potent and a little can go a really long way. To calculate the cannabinoid in your full–extract oil, use the below formula:
Total amount of oil (in mg) × % cannabinoids
After determining the amount of cannabinoid every drop contains, you can divide it into several doses that you will take in the course of the day.
Keep in mind that 1g of full-extract oil is actually quite high. If you contemplating taking high doses of cannabis, make you seek the advice of a medical professional.
5. How To Dose Topicals
- Typical dose: Depends on need and product
Topicals could be used for burns or wounds or other skin issues and usually relieve pain and inflammation. Because they do not have psychoactive effects, you cannot overdose on them.
Their labels usually have the recommended dosage which you can start with as you determine what quantity suits your issue best.
6. How To Dose Transdermal Cannabis Patches
- Typical dose: each patch has 10–20mg THC
Though they have psychoactive properties, transdermal patches are usually applied to the skin directly as well. Each patch will contain between 10-20mg of THC and you should feel the effects in about 20-60 minutes.
As with the other forms of weed, start with the lower dose and move to the stronger ones once you establish that you can handle its effects.
7. How To Dose Marijuana Concentrates
- Typical dose: 25mg per dab
BHO extracts are another form of cannabis that contain especially high cannabinoid concentrations. They are usually used in a normal vaporizer or in a dab rig. Because dabs have the characteristic of varying vastly in potency, ensure your licensed retailer or dispensary clearly labels your stash.
To illustrate the potency of this form of weed, 1g (1000mg) of shatter which has 70% THC will have 700mg THC. That is enough THC for 28 doses!
As it is quite difficult to evenly split 1g of BHO into 25mg doses, you can load tiny doses that are almost rice grain sized into your chamber using a dab tool.
What is Microdosing THC?
Consuming relatively small amounts of psychoactive chemicals is what the term “microdosing” means. In the context of tetrahydrocannabinol (THC), the term “microdosing” refers to the practice of consuming just the right amount of the compound to enjoy its positive effects, such as relaxation, greater focus, or pain relief, without experiencing the pronounced “high” or the psychoactive effects that come with larger dosages.
This means that users can go about activities of the day while medicating on cannabis.
This will also save you some weed and money at the need of the day.
How Much is a Microdose of THC?
The answer differs from person to person depending on factors such as their metabolic rate, body weight, and level of tolerance.
According to a standard measure, a microdose of THC is anywhere between 1 and 5 milligrams for the vast majority of people.
It is absolutely necessary to begin at a modest dose and gradually increase it in accordance with your individual requirements and responses.
How to Effectively Microdose Cannabis
Generally, 10mg of THC is considered a standard dose for this cannabinoid. Consequently, anything less than this amount could be considered a microdose.
That said, most people will use extremely low amounts of THC when microdosing regularly to achieve the desired effect. Typically a dose of 1-3 mg of THC is considered an effective starting dose for microdosing cannabis.
Several factors affect how THC is metabolized in the body. This includes a person’s age, gender, total body fat, and individual physiology. Additionally, factors such as tolerance, frequency of use, and method of consumption will also come into play.
All factors considered, a dose of 2mg of THC is considered an ideal starting dose for microdosing.
After starting on the lowest possible dose you should maintain for three days to give the cannabinoid enough time to start producing noticeable effects. If you see no change you can slighty increase the dose and observe for another three days. Once you find an optimal dose you can maintain that as your microdose.
It is recommended that those who have developed a level of tolerance for cannabis go on an initial two day “fast” before starting on a microdosing program.
Is Microdosing Cannabis for Everyone?
As much as microdosing cannabis has several advantages, it is not for everyone. Those who use cannabis for medicinal reasons should consult with their marijuana doctor before starting on a microdosing regimen. This is because some conditions such as the management of severe pain require high amounts of THC.
Which Cannabis Products are Ideal for Microdosing?
When selecting products for microdosing consider the concentration of THC in the product as this will make it easier to find an optimal microdose.
Remember that you are targeting doses as low as 1-3mg of THC. Putting this in perspective, capsules and gummies usually come in low doses that start from at least 5mg. So in this case you are talking about half a capsule or gummy.
Taking a puff or two of cannabis may be an effective way of microdosing. However, this method may not allow you to find the optimal dose that works best for you. The same applies to vaping cannabis.
Cannabis tinctures may be helpful for microdosing. A cannabis tincture containing 5mg of THC per mL may be the best option for microdosing.
When using cannabis edibles you should wait around 2 hours before increasing the dosage. This is because the effects take a bit longer to kick in compared to smokable forms of cannabis. On the flip side, the effects from edibles will last longer in the body.

Are There any Disadvantages of Microdosing Cannabis?
Microdosing helps to minimize the negative side effects that are associated with taking high amounts of THC. Of course it also creates efficiency going by the principle of less is more?
Perhaps the major setback with microdosing is that it may not work for recreational users since euphoria is not achieved at such low doses. If you are looking for that spacey or intoxicating feeling you are better off trying higher doses of THC.
As it stands now, the cannabis industry is obsessed with potency and a high level of THC. It means everything to so many people who consume cannabis; whether medical cannabis or recreational cannabis.
It is more difficult to practice microdosing when you smoke a joint since you have to use a certain amount of buds to build the joint.
Smoking is not the ideal choice, if you are thinking about microdosing since it is not easy to get the precise THC level.
The Benefits of Microdosing THC
There are some benefits to microdosing weed but what are the benefits exactly? For one, it helps to reduce anxiety and pain.
Many people are not aware that large doses of THC in recreational or medical cannabis can essentially cause anxiety in some.
When you smoke or consume too much cannabis, it can exacerbate your anxiety.
However, if you choose lower doses (micro-dosing), you will stand a better chance to be in a better mood without relying on psychoactive effects.
Micro-dosing weed can at times help you reduce symptoms related to depression, insomnia, inflammation, indigestion and nausea.
“
There are over 300,000 jobs in the cannabis industry. CTU trained me for one of them!

Makes $24.50 @ THC +
Micro-dosing cannabis allows you to be more focused, alert and creative. You likely won't feel overly stoned at any time nor lethargic and paranoid.
Microdosing THC For Sleep
Microdosing of THC has showed promise as an alternative to conventional sleep aids, which is encouraging news for the many people who battle with sleep difficulties.
The presence of even trace levels of THC can help induce relaxation, reduce feelings of anxiety, and make it easier to fall asleep. It is essential to speak with a healthcare practitioner and carefully monitor your dosage in order to figure out what is appropriate for your specific requirements.
Improved Creativity and Concentration
Microdosing of THC is becoming increasingly popular among creative types, such as authors, artists, and other professionals who are looking to boost their concentration and creativity.
The brain pathways can theoretically be opened up by low doses, which enables a more profound state of concentration as well as an influx of creative thoughts.
Treatment for Both Anxiety and Pain
It's possible that a microdose of THC could provide relief to people who suffer from chronic pain or anxiety.
The Ideal Strains for Marijuana Microdosing
When it comes to selecting the optimal cannabis strains for microdosing, it is essential to look for strains that have a THC to CBD ratio that is in the sweet spot. CBD can help counteract the psychoactive effects of THC, making it an excellent choice for people who wish to avoid getting high but still experience some of the benefits of cannabis.
The following are some of the most suitable strains for microdosing:
ACDC Strain
ACDC is a strain that has a high concentration of CBD but very little THC. The effects that it has on soothing and reducing pain are well-known.
Harlequin Strain
The CBD to THC ratio of Harlequin cannabis strain is 5:2, making it a well-balanced hybrid. Because it has an effect that is both energizing and stimulating, it is a wonderful choice for use throughout the afternoon.
Cannatonic Strain
Cannatonic is a strain that has a high concentration of CBD but a low level of THC. It has been shown to have a calming and pain-relieving impact on people.
Remedy Strain
The cannabis strain known as Remedy has significant levels of CBD but relatively low levels of THC. It is commonly used because of its sedative and anti-inflammatory properties.
Kits for Microdosing Marijuana
When it comes to getting started with microdosing cannabis, there are a number of different microdosing kits that are now on the market that you can choose from.
These kits often contain a variety of cannabis products with a low effective dose, such as edibles or tinctures, in addition to the accessories necessary for accurately measuring and eating the goods.
To experiment with microdosing, using one of these can be a practical and economical option.
My Personal Experience Microdosing Cannabis
As a medical cannabis professional I have a keen interest in cannabis effects. I began microdosing cannabis years ago as a way to feel the positive effects of cannabis without the over stimulating ones that todays high thc potent strains often cause. I can speak to the positive outcomes of microdosing marijuana for relief from anxiety and nausea.
Final Summary on Microdosing Cannabis
Microdosing THC is a personal decision, but one that can make you feel good without the considerable ‘high.'
Microdosing THC provides a different option for experiencing the advantages of cannabis without the intensity of a full-blown high.
Be aware, have patience, and pay attention to how your body reacts when attempting to find the optimal dosage of THC, whether you are using microdose THC gummies, researching its usage for sleep, or trying to figure out how much you should take.
Final Thoughts on CBD Dosage
CBD oil is excellent for reducing chronic pain and anxiety. However, the average CBD dose changes from one person to another. That is why you need to know how to calculate the correct dose based on your weight and what condition you are treating in your body.
There are different doses to consider for various conditions. For example, the dose for improving sleep would be different from treating chronic pain.

Karen Getchell
Karen gained expertise in developing training programs and technical documentation as a Senior Editor at Cisco Systems. She began her journey in cannabis as a patient, searching for a way to heal herself. When she perfected a method for making cannabis oil, other patients began to seek her out. An early adopter of CBD medicine, she started her CBD-infused-products business in 2014. Over the last two decades, Karen has taught hundreds of patients and caregivers how to select strains, infuse oils, and extract cannabinoids.
When she isn’t teaching cannabis cooking classes, Karen works as a cannabis business consultant, writes for online cannabis publications like Cannabis Training University, Leafly, and Weedmaps, and runs a CBD-infused-product business.











 Jeff was involved in an accident where he endured a traumatic brain injury. He had a week-long stay in ICU where brain surgeons
Jeff was involved in an accident where he endured a traumatic brain injury. He had a week-long stay in ICU where brain surgeons  100% risk free money back guarantee within 48 hours after purchase if student has not completed any of the courses or exams.
100% risk free money back guarantee within 48 hours after purchase if student has not completed any of the courses or exams.